Overview
Warning & Precautions
Product Summary
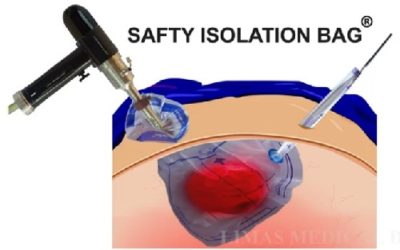
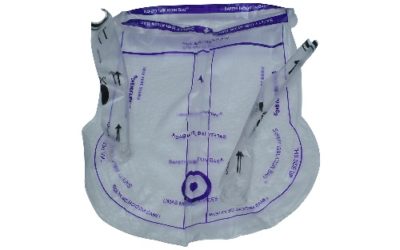
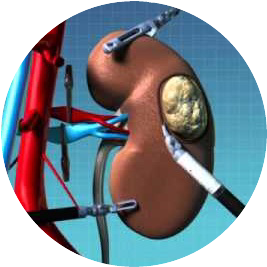
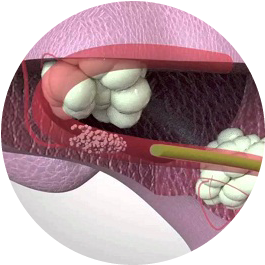
SAFETY ISOLATION BAG® the Single-use Pneumoperitoneum Device comprises of a medical grade flexible introducer with a wide mouth opening which is made up of single or multi layer construction with tubular guides ( single or double) for insertion of telescope and or other hand instruments. SAFETY ISOLATION BAG® is rolled over with a flexible introducer with a blunt tip for easy insertion of SAFETY ISOLATION BAG® through the laparoscopic port/ incision. The SAFETY ISOLATION BAG® Pneumoperitoneum Device and introducer (flexible obturator) are disposable components and are supplied sterile for one time use only. Once the device is inserted into the abdominal cavity unfold the device such that the tubular guide is kept upside. The mouth end is utilised for insertion of the pelvic masses into the device which are to be morcellated or extracted using an electromechanical morcellator. The tubular guides help the surgeons to perform the morcellation with telescope and or hand instruments through the existing incisions.
Available Sizes
Available with single or double tubular guides (multiple instruments channel) having capacity of SMALL(S) -1800ml, LARGE(L) - 2500ml, DOUBLE LARGE (LL) -3200ml, EXTRA LARGE SMALL (XLS) -4200 & EXTRA LARGE (XL) – 5000mlIntended Use
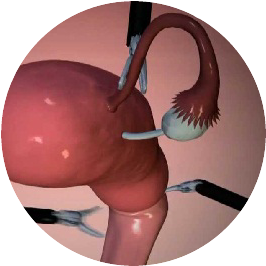
SAFETY ISOLATION BAG® is meant for use by surgeons who have been professionally trained in the use of laparoscopic power morcellator devices and laparoscopic procedures. SAFETY ISOLATION BAG® is a single-use disposable device intended to be used as a receptacle for laparoscopic removal of tissue masses during endoscopic procedures such as, ovarian cyst, laparoscopic myomectomy or laparoscopic hysterectomy etc. SAFETY ISOLATION BAG® is indicated to laparoscopically isolate the benign tissue mass such as myoma and uterus during morcellation procedure using a power morcellator by trained surgeons.
General Precautions
The Instructions for Use - manual should be thoroughly studied and ensured that all the instructions given in this user manual are followed while using the device. This product is intended for use only by clinicians with adequate training, knowledge and experience in the use of laparoscopic endoscopic procedures. The clinician is advised to consult the medical literature regarding techniques, complications and hazards associated with the intended procedures.
Failure to carefully follow all applicable instructions may result in significant injury to the patient, surgeon or attendants and may have an adverse effect on procedural outcomes. Good surgical practices should be followed for management of contaminated or infected wounds.
Use of SAFETY ISOLATION BAG® should be considered in patients whom the physician determines are not high-risk surgical candidates.
Procedural Precautions
The sharp blade of the Morcellator should be kept completely covered or in “OFF” configuration during its insertion and removal through the SAFETY ISOLATION BAG®. Insertion of SAFETY ISOLATION BAG® through the laparoscopic port, morcellation of tissue masses inside SAFETY ISOLATION BAG® and removal of SAFETY ISOLATION BAG® from the abdominal cavity should be performed under direct visualization at all times. SAFETY ISOLATION BAG® is devised to act as a Pneumoperitoneum Device, a containment receptacle during endoscopic morcellation or tissue extraction procedures of a benign pelvic tissue masses after the tissues have been detached from its stalk.
Caution should be exercised when introducing or removing sharp instruments to prevent inadvertent damage to the device. Special care should be exercised when inserting sharp or angled-edged endoscopic /laparoscopic instruments to prevent tearing of the device.
Device Related Precautions
Careful inspection of the SAFETY ISOLATION BAG® and all associated equipments prior to use is extremely essential. If the sterile packaging of SAFETY ISOLATION BAG® appears to be damaged or if the seal is opened, please DO NOT attempt to use it as the sterility of the device may have been compromised. SAFETY ISOLATION BAG® and its introducer are single use disposable devices. DO NOT re-sterilize or re-use. Do not use any part of SAFETY ISOLATION BAG® or introducer beyond the indicated expiration date. SAFETY ISOLATION BAG® is intended for only one-time use in surgery. SAFETY ISOLATION BAG® should be constantly monitored by video when used for morcellation or tissue extraction. Follow aseptic techniques, while introducing the SAFETY ISOLATION BAG® into the abdominal cavity. It is recommended to use blunt instruments for holding or pulling out the device so as to prevent damaging integrity of the product. Laparoscopic Power Morcellator should be introduced inside the SAFETY ISOLATION BAG® while in “OFF” configuration and with a clockwise spiral movement. Blunt obturator of the morcellator should always be used for while introducing a tissue morcellator into SAFETY ISOLATION BAG®
While using SAFETY ISOLATION BAG® in conjunction with exposed blades or cutting instruments, care should be taken to avoid contact with SAFETY ISOLATION BAG® as this may rupture the device. SAFETY ISOLATION BAG® should not be used with vacuum-assisted Morcellator.
After use disposal should be done in the safe manner in accordance with the applicable regulations or as per the disposal procedure followed in your healthcare institution.
Adverse Events & Potential Complications
SAFETY ISOLATION BAG® system may get ruptured during surgery and may cause spillage of tissues, blood or fluids in the patient. If this happens, the tissue could get left in the body and cause an infection, pain, or tissue growth, including cancerous growths. As with any surgical procedure, there are also risks of damage to surrounding tissues or having to convert to an open surgery (with bigger incisions).
Contraindications
SAFETY ISOLATION BAG® should not be used for morcellation or removal of tissue which is suspected or confirmed to be cancerous